
Global Pharma Contract Research Organization (CRO) Services Market Forecasts to 2024
- July, 2018
- Domain: Healthcare - Pharmaceuticals
- Get Free 10% Customization in this Report
Overview: Contract Research Organization (CRO), also sometimes referred to as clinical research organization are a key constituent of the drug development process, which offers a range of services to pharmaceutical and biopharmaceutical companies. CRO’s can provide services such as early phase development services (includes discovery studies, chemistry, manufacturing & control), Pharmacokinetics/Pharmacodynamics (PK/PD), toxicology testing, clinic research services (phase I, II, III, and IV), laboratory services, analytical and bioanalytical testing, physical characterization, and consulting services. CRO also extend their services to governmental institutes, universities, in addition to research institutions. CRO’s helps in the drug development of various major diseases such as oncology, cardiovascular disease, diabetes, infectious diseases, CNS disorders, respiratory disorders and other disorders.
The market for pharma contract research organization is driven by increasing outsourcing of research and development by pharmaceutical companies, increasing clinical trials across the globe, increasing number of patent expiration and growth in biopharmaceuticals market are some of the factors driving the growth of CRO market, whereas, lack of skilled personnel and quality issues related to services offered by CROs are limiting the growth of CRO market to an extent.
Market Analysis: The “Global Pharma Contract Research Organization market” is estimated to witness a CAGR of 8.2% during the forecast period 2018–2024. The global market is analyzed based on three segments –Service type, therapeutic area and regions.
Regional Analysis: The regions covered in the report are the North America, Europe, Asia Pacific, and Rest of the World (ROW). North America is the major shareholder in the global pharma contract research organization market, followed by Europe. Increasing growth of biopharmaceutical market, high quality standards of pharma and biopharma industry and availability of advanced technologies makes North America major shareholder of pharma CRO market. Asia Pacific is expected to grow at high CAGR during forecasted period due to increasing research activities, growing patient pool, and flexible regulatory environment for clinical trials.
Service Type Analysis: The pharma contract research organization market by service type is segmented into discovery, preclinical studies, clinical studies and others. Among these, clinical study services accounted for the highest market share in 2017, due to increasing prevalence of lifestyle and metabolic diseases, growth in the elderly population and increasing clinical trials globally.
Therapeutic Area Analysis: The market by therapeutic area is segmented into oncology, infectious disease, cardiovascular disease, CNS disorders, immunological disorders, respiratory disease, diabetes and others. Oncology occupied a major market share in 2017 and is expected to remain same for next five years. This is due to increasing global prevalence of cancer across the globe and high number of ongoing clinical trials in this segment.
Key Players:
Laboratory Corporation of America Holdings (Covance), Charles River Laboratories, Inc., Pharmaceutical Product Development Inc., IQVIA Holdings Inc., PAREXEL International Corporation, ICON plc, Syneos Health, Inc., Medpace Holdings, Inc., Envigo, Evotec AG, Eurofins Scientific, PRA Health Sciences, Inc., WuXi AppTec, Inc., SGS SA, EPS International, Genscript Biotech Corporation and niche players.
Competitive Analysis: There is increase in acquisitions and mergers by the CRO’s in recent years to gain global foothold in the highly competitive CRO market. For instance, in August 2017, LabCorp acquired CRO Chiltern for $1.2 billion, which will become part of the company’s Covance Drug Development business. This will strengthen LabCorp’s Position as a Global Life Sciences Company with Leading Diagnostics and Drug Development Businesses. In August 2017, Avista Healthcare and CRO Envigo have entered into a definitive merger agreement under which Envigo will become a wholly owned subsidiary of Avista. In August 2017, Mercachem and Syncom, two of the leading European drug discovery Contract Research Organizations (CRO), announced the merger of two companies that will result in the formation of Mercachem-Syncom Group, with more than 300 employees and operations in Nijmegen and Groningen. In addition, the company has numerous partnership to enhance their service capabilities. For instance, In March 2018, PAREXEL announced a partnership with CHA Medical Group to increase its early phase clinical development capabilities in South Korea. WuXi AppTec entered into partnership with biopharmaceutical company Antengene Corporation to accelerate drug development for oncology.
Benefits: The report provides complete details about the services offered by pharma contract research organizations in various therapeutic verticals and regions. With that, key stakeholders can know about the major trends, drivers, investments, and vertical player’s initiatives. Moreover, the report provides details about the major challenges that are going to impact on the market growth. Additionally, the report gives the complete details about the key business opportunities to key stakeholders to expand their business and capture the revenue in the specific verticals to analyze before investing or expanding the business in this market.
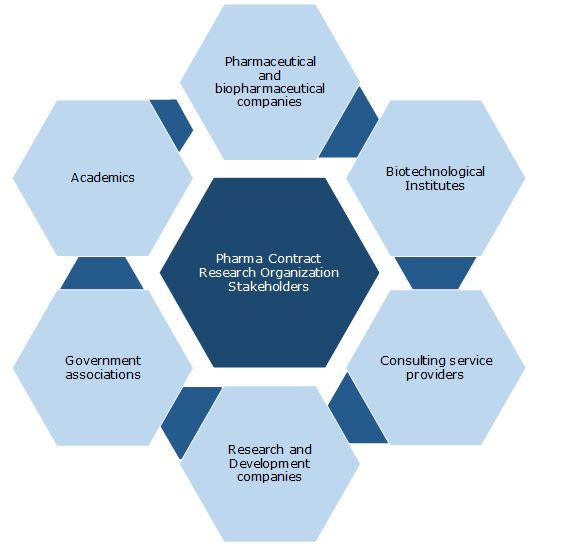
Key Stakeholders:
1 Industry Outlook
1.1 Industry Overview
1.1.1 Industry Trends
1.1.2 R&D Pipeline in Pharmaceutical Industry
1.2 Total addressable market
2 Report Outline
2.1 Report Scope
2.2 Report Summary
2.3 Research Methodology
2.4 Report Assumptions
3 Market Snapshot
3.1 Market Definition – Infoholic Research
3.2 Need of CRO
3.3 History of CRO
3.4 Segmented Addressable Market (SAM)
3.5 Trends of the Pharma Contract Research Organization Market
3.6 Related Markets
3.6.1 Pharmaceutical Contract Manufacturing Organization Market
3.6.2 Drug Discovery Outsourcing
4 Market Outlook
4.1 Funding Scenario
4.2 Process of Drug Development
4.3 Market segmentation
4.4 PEST Analysis
4.5 Porter 5 (Five) Forces
5 Market Characteristics
5.1 DRO – Global Pharma Contract Research Organization Market Dynamics
5.1.1 Drivers
5.1.1.1. Upsurge in outsourcing of R&D activities
5.1.1.2. Rising number of clinical trials across the globe
5.1.2 Opportunities
5.1.2.1. Growth in the biopharmaceutical market
5.1.2.2. Increasing number of deals to develop new compounds for rare disease
5.1.3 Restraints
5.1.3.1. Quality issues related to services offered by CRO
5.1.3.2. Lack of trained professionals
5.2 DRO – Impact Analysis
5.3 Key Stakeholders
6 Service type: Market Size & Analysis
6.1 Overview
6.2 Discovery Services
6.3 Preclinical Study Services
6.3.1 Pharmacokinetics/Pharmacodynamics
6.3.2 Toxicology Testing
6.3.3 Others
6.4 Clinical Study Services
6.4.1 Phase I
6.4.2 Phase II
6.4.3 Phase III
6.4.4 Phase IV
6.5 Others
7 Therapeutic Indication: Market Size & Analysis
7.1 Overview
7.2 Oncology
7.3 Respiratory Disease
7.4 CNS Disorder
7.5 Infectious Disease
7.6 Cardiovascular Disease
7.7 Immunological Disorder
7.8 Diabetes
7.9 Others
8 Regions: Market Size and Analysis
8.1 Overview
8.2 North America
8.3 Europe
8.4 Asia Pacific
8.5 Rest of the World
9 Competitive Landscape
9.1 Overview
10 Vendor Profiles
10.1 Charles River Laboratories, Inc.
10.1.1 Overview
10.1.2 Charles River Laboratories, Inc.: Recent Developments
10.1.3 Business Units
10.1.4 Geographic Revenue
10.1.5 Business Focus
10.1.6 SWOT Analysis
10.1.7 Business Strategies
10.2 Laboratory Corporation of America Holdings (LabCorp)
10.2.1 Overview
10.2.2 Laboratory Corporation of America Holdings: Recent Developments
10.2.3 Business Units
10.2.4 Geographic Revenue
10.2.5 Business Focus
10.2.6 SWOT Analysis
10.2.7 Business Strategies
10.3 IQVIA HOLDINGS INC.
10.3.1 Overview
10.3.2 Business Units
10.3.3 Geographic Revenue
10.3.4 Business Focus
10.3.5 SWOT Analysis
10.3.6 Business Strategies
10.4 Pharmaceutical Product Development Inc
10.4.1 Overview
10.4.2 Business Focus
10.4.3 SWOT Analysis
10.4.4 Business Strategy
10.5 ICON PLC
10.5.1 Overview
10.5.2 Geographic Revenue
10.5.3 Business Focus
10.5.4 SWOT Analysis
10.5.5 Business Strategies
10.6 PAREXEL International Corporation
10.6.1 Overview
10.6.2 Business Units
10.6.3 Geographic Revenue
10.6.4 Business Focus
10.6.5 SWOT Analysis
10.6.6 Business Strategies
11 Companies to Watch for
11.1 PRA HEALTH SCIENCES, INC.
11.1.1 Overview
11.2 Syneos Health, Inc.
11.2.1 Overview
11.3 WuXi AppTec
11.3.1 Overview
11.4 Medpace Holdings, Inc.
11.4.1 Overview
11.5 SGS SA
11.5.1 Overview
11.6 Envigo
11.6.1 Overview
11.7 EPS INTERNATIONAL
11.7.1 Overview
11.8 EVOTEC AG
11.8.1 Overview
11.9 GenScript Biotech Corporation
11.9.1 Overview
11.10 Eurofins Scientific
11.10.1 Overview
Annexure
 Abbreviations
Research Framework
Infoholic research works on a holistic 360° approach in order to deliver high quality, validated and reliable information in our market reports. The Market estimation and forecasting involves following steps:
- Data Collation (Primary & Secondary)
- In-house Estimation (Based on proprietary data bases and Models)
- Market Triangulation
- Forecasting

Market related information is congregated from both primary and secondary sources.
Primary sources
involved participants from all global stakeholders such as Solution providers, service providers, Industry associations, thought leaders etc. across levels such as CXOs, VPs and managers. Plus, our in-house industry experts having decades of industry experience contribute their consulting and advisory services.
Secondary sources
include public sources such as regulatory frameworks, government IT spending, government demographic indicators, industry association statistics, and company publications along with paid sources such as Factiva, OneSource, Bloomberg among others.
